What Does the Second Quantum Number L Describe
Thus d orbital corresponds. They can even take on.

Quantum Theory Review 1 Zinc 30 Would Have Its Final Electrons Placed In What Sublevel 1 S 2 P 3 D 4 F Table Ppt Download
Hence d orbitals have five orientations in space.

. Corresponds to spd and f subshells containing spdf. The four quantum numbers used to describe the electrons are n2 ℓ1 m1 0 or -1 and s12 the electrons have parallel spins. The magnetic quantum number of an electron is one of the four quantum numbers that state the position of the electron with respect to the nucleus.
The value of the electrons orbital is represented by the angular momentum quantum number s0 p1. The value of l depends on the value of the principle. The angular momentum quantum number determines the shape of the orbital.
What does the second quantum number l describe. SECONDARY QUANTUM NUMBER l - Represents the energy sublevel or type of orbital occupied by the electron. Give four quantum numbers that describe an electron in a 5s orbital.
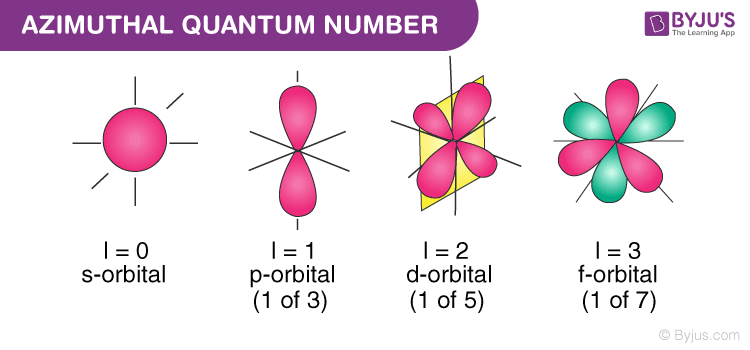
About 1914-1915 Arnold Sommerfeld realized that Bohrs n was insufficient. The number against an orbital is indicated by the energy level of the electron in that orbital. The angular momentum quantum number l also referred to as the secondary quantum number or azimuthal quantum number describes the shape of the orbital that an.
The second quantum number known as the angular or orbital quantum number describes the subshell and gives the magnitude of the orbital angular momentum through the relation. L along with n and the third quantum. What is the second quantum number of a 1s2 electron in phosphorus 1s22s22p63s23p3.
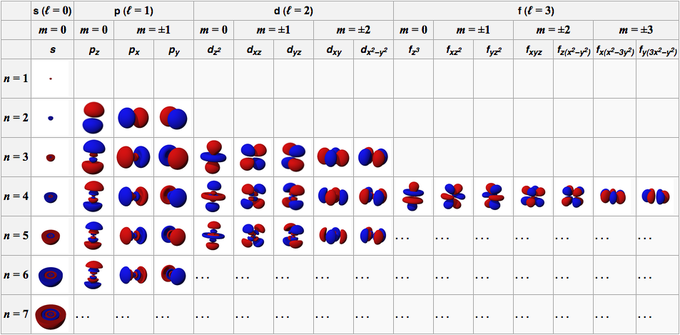
A value of l0 corresponds to s l1 is p l2 is d and so forth. Magnetic Quantum Number Definition. The value of l depends on the value of n such that l 0 1.
The third is magnetic quantum number m. Quantum Numbers Principal Azimuthal Magnetic and Spin - The set of numbers used to describe the position and energy of the electron in an atom are called quantum numbers. The angular momentum quantum number l is an integer that has possible values of 0 1 2 3 etc.
What are the four different energy. Specifies the shape of an orbital with a particular principal quantum number. I dont know this one Also I have another question that says to give the.
The angular quantum number l describes the shape of the orbital. Angular Momentum Secondary Azimunthal Quantum Number l. What is the second quantum numberI.
The second quantum number or l which describes the subshell s p d f etc. The energy level closest. Quantum numbers are a set of variables which describe pertinent characteristics of electrons within an atom.
The second number indicates the sublevel - can only be numbers 0-3 and must be smaller than the first number. Second Quantum Number. Electrons can be situated in one.
Not Just for Electrons While quantum numbers. N 5 l 0 ml 0 ms. L 0 n-1.
The second quantum number is the angular quantum number ℓ. The angular momentum quantum number signified as l describes the general shape or region an electron occupiesits orbital shape. Each value of n has multiple values of ℓ ranging in values from 0 to n-1This.
Magnetic Quantum Number left m_l right The magnetic quantum number signified as m_l describes the orbital orientation in space. The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital. For d orbital Azimuthal quantum number l 2 and the magnetic quantum number m -2 -1 0 1 2.
Each orbital is denoted by three quantum numbers n l and m. In other words more. Orbitals have shapes that are best described as spherical l 0 polar l 1 or cloverleaf l 2.
The angular momentum quantum number l describes the shape of the subshell and its orbitals where l 0123. L is less than or equal to n-1 and bigger than or equal to zero. The Azimuthal or Angular Momentum Quantum Number signified by the letter ℓ.
The second quantum number is known as l.

Comments
Post a Comment